A well-organized business thrives on consistent quality. Quality Management System (QMS) defines an organization's processes, procedures, and responsibilities for achieving quality policies and objectives. In this post you get a complete guide on what is Quality Management System that ensures with products or services consistently meet customer and regulatory requirements. It is a systematic approach to managing quality embedded throughout an organization's operations.
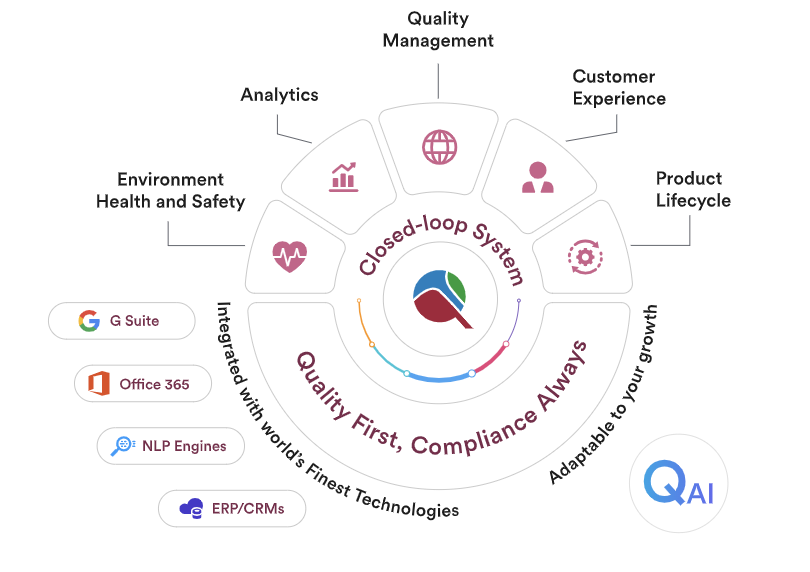
Qualityze offers cloud-based, scalable, flexible, and AI-powered QMS to modernize the quality management process. It transforms traditional, often manual, quality processes into streamlined, automated workflows.
What is a Quality Management System?
A Quality Management System (QMS) is a formalized system that documents processes, procedures, and responsibilities. These elements help achieve quality policies and objectives. A QMS assists organizations in meeting customer and regulatory requirements and improves efficiency and consistency. The system covers all aspects of an organization's operations, including planning, production, and delivery.
Why is Quality Management System Important?
A Quality Management System is crucial for modern businesses. It provides a framework for consistent operations. Here are key reasons for its importance:
- Ensures Customer Satisfaction: A QMS focuses on meeting customer needs, leading to higher satisfaction and loyalty.
- Improves Product/Service Quality: Standardized processes reduce errors, resulting in better products or services.
- Enhances Operational Efficiency: A QMS identifies inefficiencies. It streamlines workflows, saving time and resources.
- Facilitates Regulatory Compliance: Many industries have strict regulations. A QMS helps organizations comply with these rules, helping avoid penalties and legal issues.
- Boosts Employee Morale: Clear processes and training empower employees. They understand their roles in maintaining quality.
- Supports Continuous Improvement: A QMS encourages regular reviews and updates, promoting a culture of ongoing enhancement.
- Reduces Costs: Fewer defects mean less rework and waste, directly impacting the bottom line.
- Increases Market Competitiveness: Businesses with a strong QMS gain a competitive edge, demonstrating a commitment to excellence.
Benefits of Quality Management Systems
Implementing a Quality Management System offers numerous advantages. These benefits impact various aspects of an organization.
- Improved Product Reliability: Consistent processes lead to more dependable products. Customers trust reliable goods.
- Enhanced Process Efficiency: Standardizing tasks reduces variability, making operations smoother and faster.
- Stronger Customer Loyalty: Meeting customer expectations builds trust. Loyal customers become repeat buyers.
- Reduced Operational Costs: Minimizing errors and waste lowers expenses, boosting profitability.
- Better Risk Management: Identifying and addressing potential issues early prevents major problems and protects the business.
- Increased Regulatory Compliance: Adhering to standards like ISO 9001 demonstrates commitment. It also helps meet legal requirements.
- Greater Employee Engagement: Employees understand their roles in quality.
- Data-Driven Decision Making: A QMS provides data for analysis, allowing for informed decisions and strategic planning.
- Positive Brand Reputation: A commitment to quality enhances a company's image. This attracts new customers and talent.
- Facilitated Market Expansion: A strong QMS often opens doors to new markets. Many international markets require certified QMS.
What are the Core Quality Management System Processes?
A QMS involves several core processes. These processes work together to ensure quality throughout an organization.
Process Area
Description
Planning and Policy
Defines quality objectives and policies. Establishes the scope and framework of the QMS. Involves top management commitment.
Resource Management
Manages human resources, infrastructure, and work environment. Ensures adequate training and competent personnel. Provides necessary tools and facilities.
Product/Service Realization
Covers all stages from design to delivery. Includes design and development, purchasing, production, and service provision. Focuses on control and consistency.
Measurement, Analysis, and Improvement
Monitors and measures QMS performance. Conducts internal audits and analyzes data. Implements corrective and preventive actions for continuous improvement.
What are the Benefits of Implementing QMS Software?
Implementing Quality Management System (QMS) software significantly enhances an organization's quality initiatives. It moves beyond manual processes.
- Automated Workflows: Software automates routine tasks, reducing manual effort and potential errors.
- Centralized Documentation: All quality-related documents reside in one secure location, improving accessibility and version control.
- Enhanced Data Visibility: Dashboards and reports provide real-time insights. Decision-makers access critical quality data quickly.
- Improved Collaboration: Teams can easily share information and track progress, promoting a more collaborative environment.
- Streamlined Audits: QMS software simplifies audit preparation and execution. It provides a clear trail of documented processes.
- Faster Issue Resolution: Automated alerts and tracking mechanisms speed up the identification and resolution of non-conformances.
- Better Compliance Management: The software helps organizations maintain compliance with various standards. It tracks regulatory requirements.
- Reduced Human Error: Automation minimizes mistakes common in manual data entry, leading to more accurate records.
- Scalability and Flexibility: QMS software adapts to business growth. It supports evolving quality management needs.
Key Features to Consider When Choosing QMS Software
Selecting the right QMS software is crucial. It requires evaluating several key features.
- Document Control: Enterprise Document Management Software must manage all quality documents, including version control, approval workflows, and secure storage.
- Audit Management: It should facilitate planning, conducting, and reporting internal and external audits. Look for features like audit checklists and CAPA integration.
- Non-Conformance Management: The system must efficiently handle non-conformances, including logging, investigation, root cause analysis, and corrective/preventive actions (CAPA).
- Training Management: The software should track employee training records. It ensures employees are qualified for their roles.
- Complaint Management: A robust system processes customer complaints systematically. It ensures timely resolution and prevents recurrence.
- Supplier Quality Management: The software should help manage supplier approvals and performance. It tracks supplier non-conformances.
- Risk Management: Integrated risk assessment tools help identify and mitigate potential quality risks, supporting proactive quality planning.
- Reporting and Analytics: The software must offer comprehensive reporting capabilities. It provides actionable insights into quality performance.
- Integration Capabilities: It should integrate with other business systems. This includes ERP, CRM, or LIMS.
- User-Friendliness: An intuitive interface promotes user adoption. It minimizes training time.
- Scalability: The software needs to grow with your organization. It supports increasing data volumes and users.
- Regulatory Compliance: Ensure the software meets industry-specific regulatory requirements, including FDA, ISO, or other standards.
- Security Features: Data security is paramount. The software must protect sensitive quality information.
- Customer Support: Reliable vendor support is essential. It assists with implementation and ongoing use.
Industries that Benefit from Qualityze EQMS Software
Qualityze EQMS Software offers significant advantages across diverse industries. Its robust features cater to specific regulatory and operational needs.
- Pharmaceutical: Strict regulations govern drug development and manufacturing. Qualityze helps manage clinical trials, documentation, and regulatory submissions (e.g., FDA 21 CFR Part 11 compliance).
- Biotechnology: This sector requires rigorous control over research, development, and production processes. Qualityze assists with lab management, quality control, and compliance.
- Medical Devices: Manufacturers face stringent quality and safety standards. The software supports design control, risk management, and device complaint handling.
- Food and Beverage: Ensuring product safety and compliance with food regulations is critical. Qualityze helps manage HACCP plans, supplier quality, and allergen control.
- Manufacturing: From automotive to aerospace, manufacturing relies on consistent product quality. Qualityze streamlines production quality, defect tracking, and supplier management.
- Life Sciences: This broad sector encompasses various industries with high regulatory demands. Qualityze EQMS for lifescience inductry provides a comprehensive solution for compliance and quality assurance.
- Cannabis: This emerging industry requires meticulous tracking and quality control. Qualityze supports seed-to-sale tracking and compliance with evolving regulations.
Conclusion
A Quality Management System is a strategic asset for any business. It ensures consistent product or service quality, boosts customer satisfaction, and drives operational efficiency. Modern QMS software, like Qualityze EQMS Suite, digitizes and streamlines these critical processes. This transformation leads to increased efficiency, better compliance, and data-driven decision-making. By embracing a robust QMS, businesses effectively navigate today's complex market demands. They achieve sustainable growth and build a strong reputation for excellence.




Comments