Safety Needles Industry
Summary:
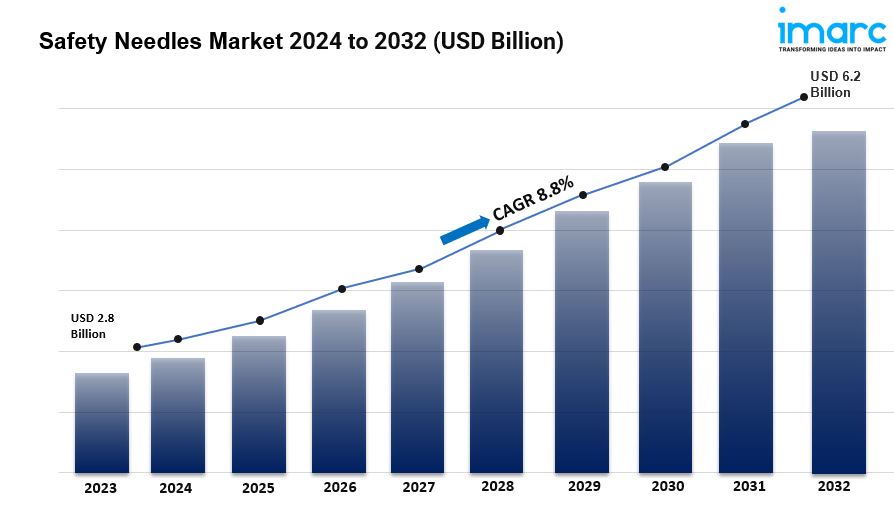
- The global safety needles market size reached US$ 2.8 Billion in 2023.
- The market is expected to reach US$ 6.2 Billion by 2032, exhibiting a growth rate (CAGR) of 8.8% during 2024-2032.
- Region-wise the market has been segmented into North America (United States and Canada); Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others); Europe (Germany, France, United Kingdom, Italy, Spain, Russia, and others); Latin America (Brazil, Mexico, and others); and Middle East and Africa.
- Based on the product, the market has been divided into active safety needles, passive safety needles, and others.
- On the basis of application, the market has been classified into drug delivery, sample collection, and injection.
- Based on the end user, the market has been categorized into hospitals and ambulatory surgery centers, diabetic patients, family practices, psychiatry, and others.
- The rising incidence of needlestick injuries across the globe is a primary driver of the safety needles market.
- The expanding healthcare sector and the implementation of strict regulatory mandates are further reshaping the safety needles market.
Industry Trends and Drivers:
- Growing Incidence of Needlestick Injuries:
One of the primary factors driving the safety needles market is the increasing occurrence of needlestick injuries among healthcare workers. These injuries, caused by accidental punctures from contaminated needles, pose a significant risk of transmitting bloodborne pathogens such as hepatitis B, hepatitis C, and human immunodeficiency virus (HIV). This growing concern has raised awareness among medical professionals and healthcare organizations about the necessity of adopting safety measures, including the use of safety needles. Traditional needles without protective mechanisms increase the risk of such injuries, especially in high-pressure environments where time and workload constraints can lead to accidents. As a result, hospitals, clinics, and other healthcare facilities are increasingly turning to safety-engineered needles that include features like needle shields and retractable mechanisms, which are designed to minimize the risk of accidental injury.
- Regulatory Mandates for Safer Medical Devices:
Regulatory bodies worldwide have played a pivotal role in promoting the use of safety needles by implementing stringent guidelines and policies aimed at enhancing healthcare worker safety. These regulations not only apply to hospitals and clinics but also to any environment where needles are used, including laboratories and outpatient care facilities. The regulatory push has created a mandatory shift toward safety needles in medical practice, accelerating market growth as organizations seek compliance with these guidelines. This also leads to increased demand for manufacturers to supply safety needles that meet specific safety standards, ensuring that healthcare providers are equipped with the right tools to minimize risks. Moreover, penalties and fines for non-compliance with these mandates further incentivize institutions to adopt safety needles.
- Growing Healthcare Sector and Surgical Procedures:
The expanding healthcare industry and the rising number of surgical procedures globally are also major contributors to the growing demand for safety needles. With the aging population, there is an increasing prevalence of chronic diseases such as diabetes, cardiovascular diseases, and cancer, all of which require regular medical interventions involving injections and blood draws. This surge in the number of injections being administered has resulted in greater reliance on needles, making the need for safety-enhanced devices crucial. Additionally, vaccinations and immunization programs have further amplified the demand for needles and syringes. The safety concerns tied to frequent needle use in such scenarios make the adoption of safety needles a priority for healthcare providers. Furthermore, as medical technology advances, the number of minimally invasive surgical procedures continues to rise, all of which involve the use of needles.
Request for a sample copy of this report: https://www.imarcgroup.com/safety-needles-market/requestsample
Safety Needles Market Report Segmentation:
Breakup By Product:
- Active Safety Needles
- Passive Safety Needles
- Others
Based on the product, the market has been divided into active safety needles, passive safety needles, and others.
Breakup By Application:
- Drug Delivery
- Sample Collection
- Injection
On the basis of application, the market has been classified into drug delivery, sample collection, and injection.
Breakup By End User:
- Hospitals and Ambulatory Surgery Centers
- Diabetic Patients
- Family Practice
- Psychiatry
- Others
Based on the end user, the market has been categorized into hospitals and ambulatory surgery centers, diabetic patients, family practices, psychiatry, and others.
Breakup By Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
Region-wise the market has been segmented into North America (United States and Canada); Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others); Europe (Germany, France, United Kingdom, Italy, Spain, Russia, and others); Latin America (Brazil, Mexico, and others); and Middle East and Africa.
Top Safety Needles Market Leaders:
The safety needles market research report outlines a detailed analysis of the competitive landscape, offering in-depth profiles of major companies.
Some of the key players in the market are:
- Braun Melsungen AG
- Becton Dickinson and Company
- Boston Scientific Corporation
- Cardinal Health Inc.
- Johnson & Johnson
- Nipro Corporation
- Novo Nordisk A/S
- Retractable Technologies Inc.
- Smiths Group plc
- Terumo Corporation
- Vygon
Browse full report with TOC & List of Figures: https://www.imarcgroup.com/request?type=report&id=4215&flag=C
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145




Comments