Roots Analysis has announced the addition of “Viral Clearance and Testing Services Market, 2023 – 2035” report to its list of offerings
Key Market Insights
§ Several service providers, across the globe, have the necessary capabilities to offer viral clearance and testing services for the detection and removal of various enveloped and non-enveloped viruses
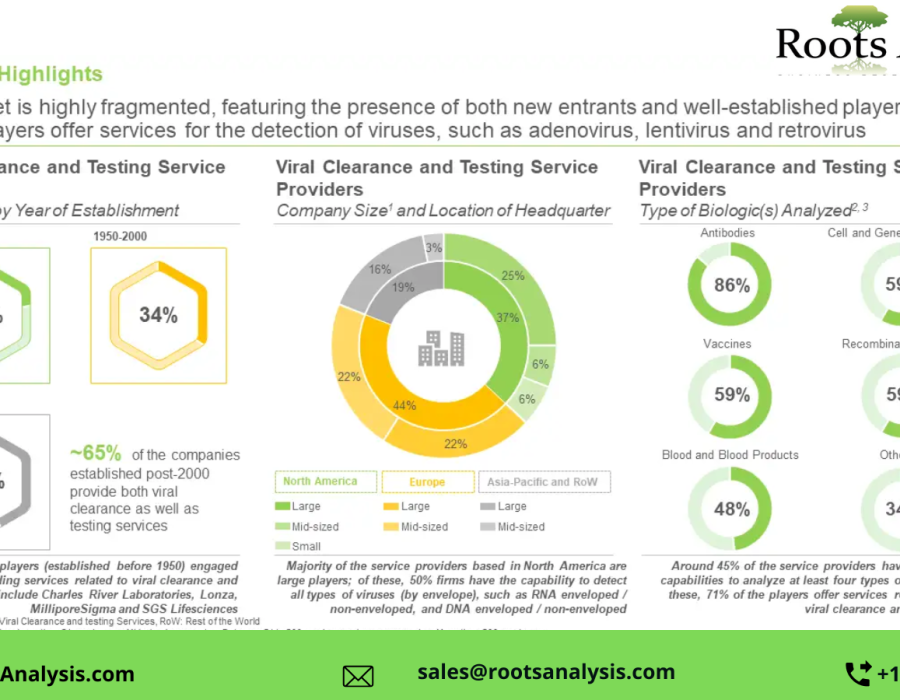
§ The market is highly fragmented, featuring the presence of both new entrants and well-established players; close to 35% players offer services for the detection of viruses, such as adenovirus, lentivirus and retrovirus
§ With around 75 facilities, viral clearance and testing service providers have established global presence; majority of these players are based in Europe, primarily in countries, such as the UK, Germany and France
§ In pursuit of building a competitive edge, stakeholders are actively upgrading their existing capabilities and enhancing their respective service offerings to comply with the evolving industry benchmarks
§ To protect the intellectual property generated within viral clearance and testing field, both industry and non-industry players have filed / were granted close to 260 patents in the past five years
§ Over the past few years, several recent expansions and partnership activities have taken place in order to meet the increasing demand for viral clearance and testing services, worldwide
§ The Viral Clearance market is projected to grow at a CAGR of ~10%, till 2035; the forecasted opportunity is likely to be distributed across different scales of operation, methods of viral clearance testing, end users, and geographical regions
Table of Content
1. PREFACE
1.1. Chapter Overview
1.2. Key Market Insights
1.3. Scope of the Report
1.4. Research Methodology
1.5. Frequently Asked Questions
1.6. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
3.1. Chapter Overview
3.2. Viral Contamination in Biologics
3.3. Need for Viral Clearance and Testing
3.4. Process of Viral Clearance and Testing
3.5. Regulatory Guidelines Related to Viral Contamination
3.6. Future Perspectives
4. VIRAL CLEARANCE AND TESTING SERVICE PROVIDERS: MARKET LANDSCAPE
4.1. Chapter Overview
4.2. Viral Clearance and Testing Service Providers: Overall Market Landscape
4.2.1. Analysis by Year of Establishment
4.2.2. Analysis by Company Size
4.2.3. Analysis by Location of Headquarters
4.2.4. Analysis by Company Size and Location of Headquarters (Region)
4.2.5. Analysis by Location of Viral Clearance and Testing Facilities
4.2.6. Analysis by Type of Virus Detected (by Envelope)
4.2.7. Analysis by Type of Virus Detected (by Class)
4.2.8. Analysis by Key Offerings
4.2.9 Analysis by Type of Biologic(s) Analyzed
4.2.10 Analysis by Method(s) of Viral Clearance (Inactivation)
4.2.11 Analysis by Method(s) of Viral Clearance (Removal)
4.2.12 Analysis by Type of Viral Testing Service(s) Offered
5. COMPANY COMPETITIVENESS ANALYSIS
5.1. Chapter Overview
5.2 Methodology and Key Parameters
5.3. Viral Clearance and Testing Service Providers in North America
5.4. Viral Clearance and Testing Service Providers in Europe
5.5. Viral Clearance and Testing Service Providers in Asia-Pacific and Rest of the World
6. VIRAL CLEARANCE AND TESTING SERVICE PROVIDERS IN NORTH AMERICA
6.1. Chapter Overview
6.2. Charles River Laboratories
6.2.1. Company Overview
6.2.2. Financial Information
6.2.3. Viral Clearance and Testing Related Services
6.2.4. Recent Developments and Future Outlook
6.3. Microbac Laboratories
6.3.1. Company Overview
6.3.2. Viral Clearance and Testing Related Services
6.3.3. Recent Developments and Future Outlook
6.4. Nelson Labs
6.5. Pall Corporation
7. VIRAL CLEARANCE AND TESTING SERVICE PROVIDERS IN EUROPE AND ASIA-PACIFIC
7.1. Chapter Overview
7.2. Eurofins Scientific
7.3. Syngene International
7.4. Texcell
8. PATENT ANALYSIS
8.1. Chapter Overview
8.2. Scope and Methodology
8.3. Viral Clearance and Testing Services Market: Patent Analysis
8.4. Viral Clearance and Testing Services Market: Patent Benchmarking Analysis
8.5. Viral Clearance and Testing Services: Patent Valuation Analysis
8.6. Leading Patents by Number of Citations
9. RECENT DEVELOPMENTS
9.1 Chapter Overview
9.2. Partnerships and Collaborations
9.3. Recent Expansions
10. VIRAL CLEARANCE AND TESTING SERVICES: MARKET FORECAST AND OPPORTUNITY ANALYSIS
10.1. Chapter Overview
10.2. Forecast Methodology and Key Assumptions
10.3. Global Viral Clearance and Testing Services Market, 2023-2035
11. CONCLUSION
12. EXECUTIVE INSIGHTS
13. APPENDIX 1: TABULATED DATA
14. APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/viral-clearance-and-testing-services-market.html
News article
Global Cell Free Expression Market
Learn from experts: do you know about these emerging industry trends?
mRNA Therapeutics and mRNA Vaccines Industry: Current Scenario and Future Trends
Novel Cell Cytometers: Need of the Hour
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Learn more about Roots Analysis consulting services:
Roots Analysis Consulting - the preferred research partner for global firms
Contact:
Ben Johnson
+1 (415) 800 3415




Comments