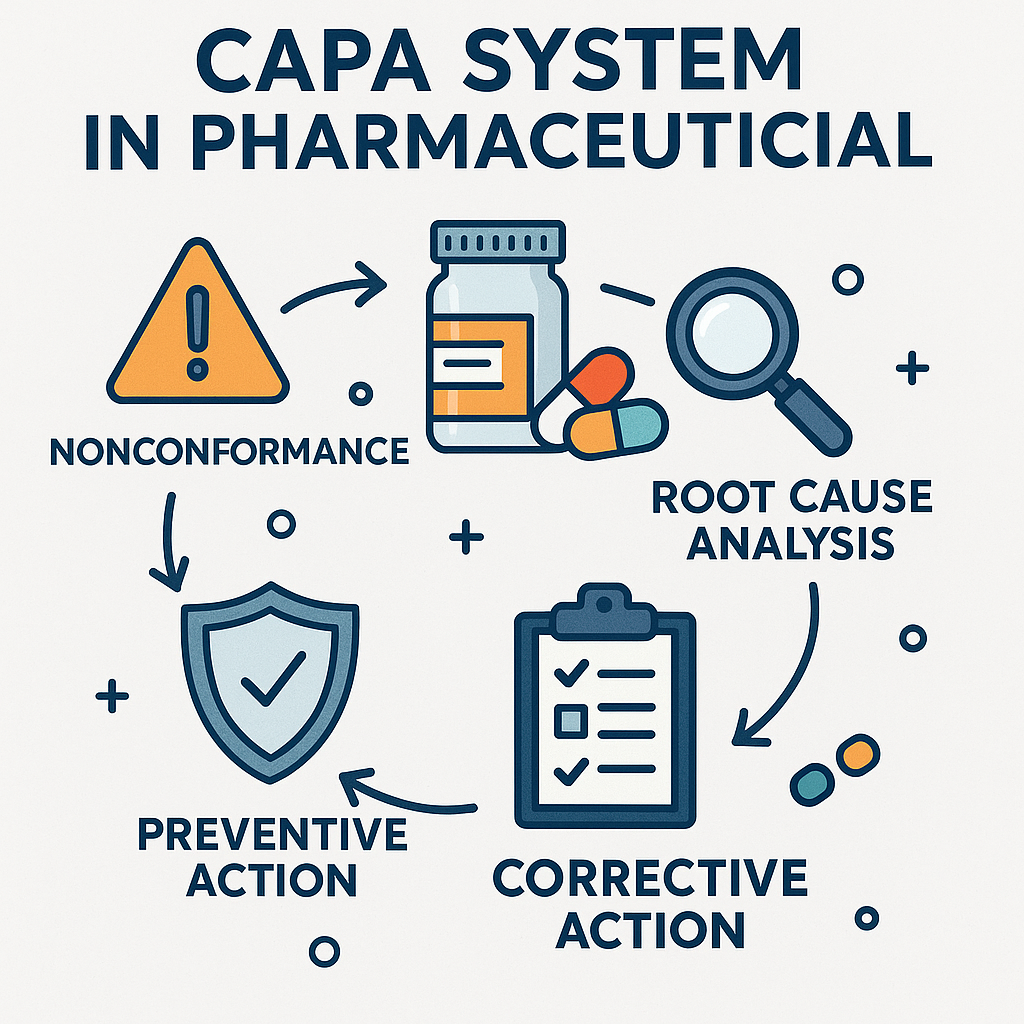
In the pharmaceutical industry, the stakes for product quality, patient safety, and regulatory compliance are exceptionally high. A CAPA system in pharmaceutical environments is essential for addressing deviations, implementing corrective and preventive actions, and establishing a continuous improvement culture across organizations. Corrective and preventive actions (CAPA) are critical for resolving quality issues, preventing recurrence, and ensuring operational excellence. By integrating CAPA management within pharmaceutical quality systems, companies can align process improvements with regulatory expectations while driving a culture of proactive quality assurance.
The industry faces increasing scrutiny from global regulatory bodies, and the complexity of multi-site operations amplifies the need for a structured CAPA system. Linking CAPA to continuous improvement provides a strategic framework that promotes operational efficiency, compliance, and organizational learning.
CAPA System in Pharmaceutical Workflows
Embedding CAPA into Quality Operations
A capa system in pharmaceutical operations is most effective when fully integrated into quality workflows. Deviations and quality incidents should automatically trigger CAPA processes, enabling timely identification of root causes and implementation of corrective and preventive actions. This ensures that CAPA management is proactive rather than reactive, supporting long-term compliance and product quality.
Streamlining Multi-Site Consistency
For pharmaceutical manufacturers operating across multiple sites, CAPA systems standardize responses to quality deviations. Centralized CAPA management ensures that corrective and preventive actions are applied consistently across locations, fostering uniform quality standards and adherence to regulatory requirements globally.
Corrective and Preventive Actions as Drivers of Continuous Improvement
Root Cause Analysis for Sustainable Solutions
Effective CAPA management focuses on uncovering the root causes of deviations instead of merely addressing the symptoms. Pharmaceutical quality systems facilitate structured investigations, ensuring that corrective and preventive actions address process gaps, equipment failures, or procedural deficiencies that could compromise product integrity.
Translating CAPA into Process Optimization
CAPA management contributes to continuous improvement by converting insights from deviations into actionable process enhancements. Preventive measures derived from CAPA analysis reduce the likelihood of recurring issues, improve efficiency, and optimize quality performance throughout the manufacturing lifecycle.
Integrating CAPA with Other Quality Processes
Harmonizing CAPA with Deviation and Complaint Management
A unified approach links CAPA to other critical quality processes, including deviation management and customer complaint handling. Integration ensures that all quality events feed into CAPA management, enabling comprehensive analysis, effective corrective actions, and continuous quality improvement across pharmaceutical operations.
Alignment with Document Control and Training
Corrective and preventive actions often require updates to standard operating procedures (SOPs) and employee training. CAPA management integrated with document control and training modules ensures that personnel have access to the latest information and guidelines, enhancing compliance and reducing the risk of human error.
Data-Driven CAPA Management
Leveraging Analytics for Quality Insights
Pharmaceutical quality systems enable robust data tracking and analytics within CAPA management. Historical CAPA data provides insight into recurring trends, common failure points, and areas needing process improvement. This analytical approach allows organizations to prioritize corrective and preventive actions that have the most significant impact on quality outcomes.
Predictive CAPA for Risk Mitigation
Advanced CAPA management systems support predictive capabilities, allowing quality teams to anticipate potential deviations and implement preventive measures proactively. By identifying risks before they escalate, pharmaceutical companies can safeguard product quality, enhance patient safety, and maintain compliance across global operations.
Enhancing Multi-Site and Global CAPA Oversight
Centralized Tracking and Reporting
Centralized CAPA management ensures visibility across all manufacturing sites. Quality leaders can monitor CAPA initiation, track progress, and measure effectiveness in real-time, enabling consistent enforcement of corrective and preventive actions across geographies.
Facilitating Regulatory Compliance
Regulatory agencies require evidence that CAPA processes are effective and consistently applied. A centralized system provides audit-ready documentation, demonstrating comprehensive CAPA investigations, root cause analysis, and closure of corrective and preventive actions, which strengthens compliance with FDA, EMA, and other global regulators.
Linking CAPA to Continuous Improvement Culture
Promoting a Quality-First Mindset
Embedding a CAPA system in pharmaceutical organizations cultivates a culture of continuous improvement. Employees view deviations as opportunities for learning rather than punitive action. This mindset encourages proactive reporting, collaborative problem-solving, and engagement in quality initiatives.
Driving Organizational Excellence
CAPA management supports continuous improvement by converting lessons learned into tangible operational improvements. Analyzing CAPA outcomes informs process redesigns, equipment upgrades, and procedural refinements, leading to increased efficiency, reduced risk, and sustained high-quality performance.
Technology Enablement for CAPA Management
Role of Pharmaceutical QMS Software
Modern pharmaceutical quality systems offer automation, real-time tracking, and analytics capabilities for CAPA management. Automated workflows reduce manual errors, streamline root cause investigations, and ensure timely implementation of corrective and preventive actions, accelerating continuous improvement initiatives.
Traceability and Accountability
QMS software in pharma provides full traceability of CAPA processes, tracking responsibilities, deadlines, and progress from initiation to closure. This ensures accountability at every stage and strengthens both operational efficiency and audit readiness.
Conclusion: Why ComplianceQuest is Essential for Business in 2025
Integrating a capa system in pharmaceutical environments with continuous improvement practices is critical for ensuring quality, compliance, and operational excellence. ComplianceQuest provides a cloud-based platform that unifies CAPA management with deviations, document control, and training modules, enabling pharmaceutical companies to implement corrective and preventive actions effectively.
With ComplianceQuest, organizations gain real-time insights into quality trends, strengthen regulatory compliance, and foster a culture of continuous improvement. For pharmaceutical companies aiming to maintain competitive advantage and achieve operational excellence in 2025 and beyond, ComplianceQuest is an indispensable solution for CAPA management and quality system enhancement.




Comments