Hepatitis D virus (HDV), also known as Delta virus, is an RNA virus that infects hepatocytes and causes hepatitis D. HDV is classified as the genus Deltavirus, within the realm Ribozyviria. Transmission of HDV can occur either via simultaneous infection with HBV (coinfection) or superimposed on chronic hepatitis B or hepatitis B carrier state (superinfection). HDV infecting a person with chronic hepatitis B (superinfection) is considered the most serious type of viral hepatitis due to its severity of complications. At least 12 million individuals infected with hepatitis B virus (HBV) are coinfected with hepatitis D virus (HDV) and have a high risk to develop liver cirrhosis and hepatocellular carcinoma within a few years. In combination with hepatitis B virus, hepatitis D has the highest fatality rate of all the hepatitis infections, at 20%.
HDV virion is a small, spherical, enveloped particle with a 36 nm diameter (Figure 1); HDV virion has a ribonucleoprotein (RNP) complex inside and an HBV derived envelope outside. The envelope contains three HBV envelope proteins: small-HBsAg (S-HBsAg), medium-HBsAg (M-HBsAg) and large-HBsAg (L-HBsAg). The RNP contains the genome surrounded by about 200 molecules of hepatitis D antigen (HDAg) for each genome. The viral genome is a 1.7 kilobase single-stranded circular RNA, and is approximately 70% complementary to itself and forms a rod-like structure, which is quite unique for animal virus. HDAg comes in two forms; a 27kDa large-HDAg, and a small-HDAg of 24kDa. The N-terminals of the two forms are identical, they differ by 19 more amino acids in the C-terminal of the large HDAg. S-HDAg is required for the initiation of the viral genome replication; it is essential for the transcription and accumulation of newly processed HDV RNAs, whereas L-HDAg, which is synthesized in the late stage of viral replication, serves as a principal inhibitor of replication and is essential in the assembly of HDV virions.
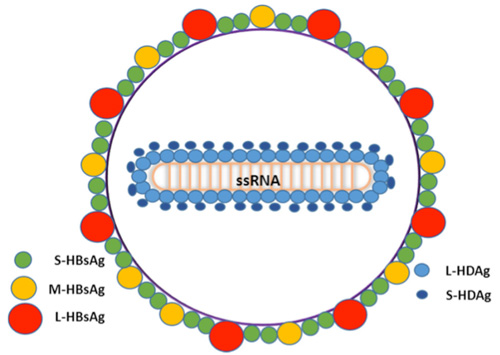
Fig.1 Structure of Hepatitis D virus1
HDV and HBV must infect hepatocytes at the same time to complete an infection cycle, because the formation of new virus particles requires HBV surface antigen. HDV first attaches to heparan sulfate proteoglycans (HSPG), and then recognizes its receptor NTCP into host cells through the N-terminal domain of hepatitis B large surface antigen HBsAg. After membrane fusion, the ribonucleoprotein (RNP) is released and further transported to the nucleus to initiate RNA replication. After membrane fusion, the ribonucleoprotein (RNP) is released and further transported to the nucleus mediated by signals in the HDAg amino acid sequence to start RNA replication. To replicate its genome, the virus uses the host RNA polymerases. Once inside the nucleus, cellular RNA polymerases synthesize the antigenomic RNA in the nucleolus and genomic RNA in the nucleoplasm. Viral protein translation occurs in the cytoplasm, and newly synthesized S-HDAg and L-HDAg (intact and prenylated) are transported into the nucleus to regulate virus replication or bind to the HDV RNA to form RNP. The RNP can be exported to the cytoplasm and encapsulated into HBV envelope through the interaction between L-HDAg and S-HBsAg. HDV virions are released through the ER-Golgi secretory pathway.
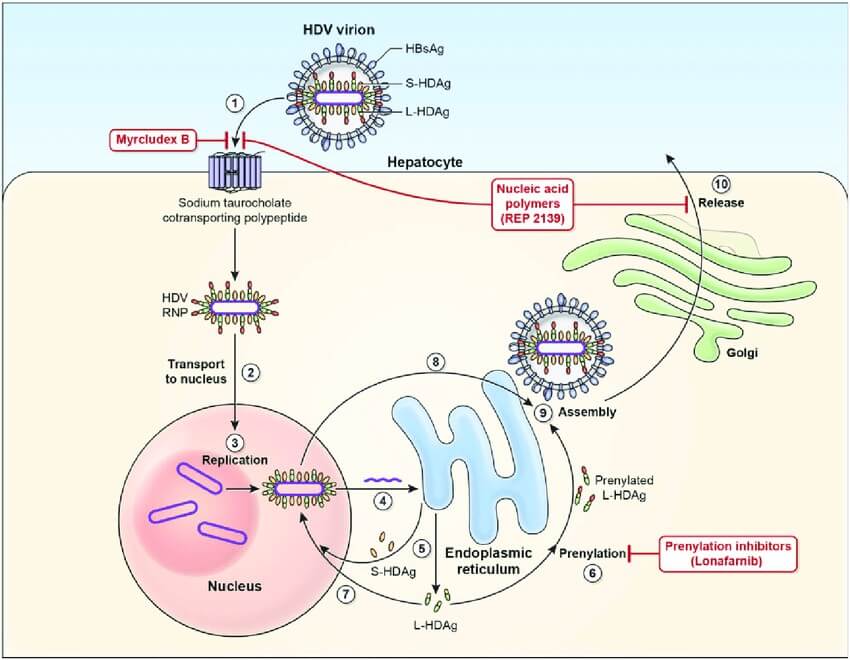
Fig.2 Hepatitis D virus viral life cycle2
With state-of-art facilities and experienced science teams, Creative Diagnostics now provides both in stock and customized high-quality Hepatitis D virus Antigens products to support your biological researches.
References
- Aldabe, R.; et al. (2015). Animal Models of Chronic Hepatitis Delta Virus Infection Host–Virus Immunologic Interactions. Pathogens, 4(1), 46–65.
- Da, B. L.; et al. (2019). Hepatitis D infection: from initial discovery to current investigational therapies. Gastroenterology Report, 7(4), 231–245.




Comments