Roots Analysis has done a detailed study on Exosome Therapeutics Market, 2022-2040, covering key aspects of the industry and identifying future growth opportunities
Key Market Insights
§ Close to 60 players, worldwide, have taken initiatives to develop exosome therapeutics; the market is characterized by the presence of start-ups and small companies
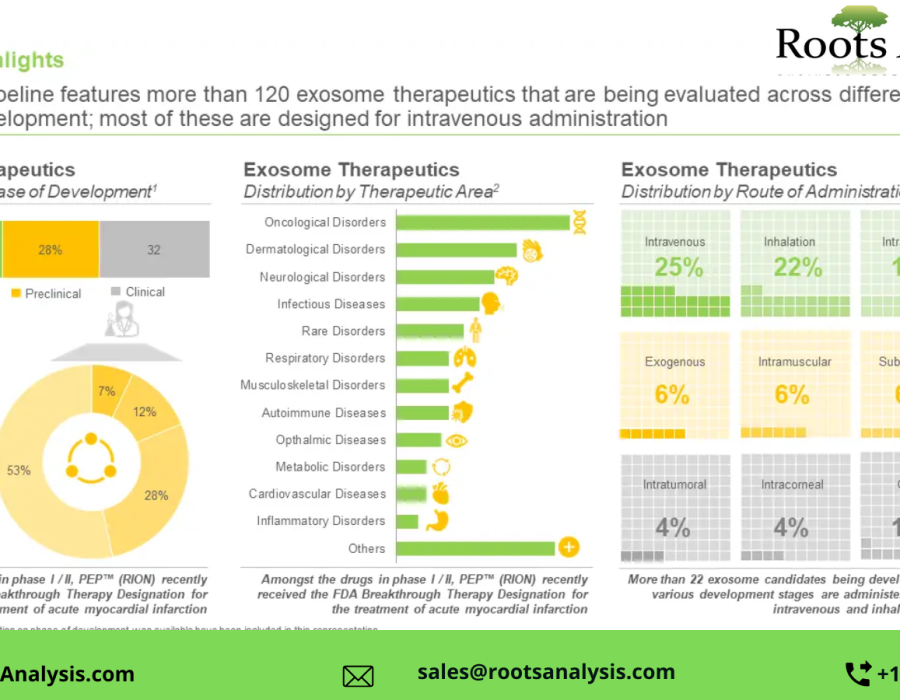
§ The current pipeline features more than 120 exosome therapeutics that are being evaluated across different phases of development; most of these are designed for intravenous administration
§ 3,000+ patients have been recruited / enrolled in clinical trials evaluating exosome related therapies and biomarkers across different geographies
§ More than 530 grants have been awarded for the ongoing R&D efforts for exosome therapeutics; University of California has been awarded the maximum grant amount of USD 21 million
§ A variety of investors, having realized the benefits and future opportunities in this field, have invested more than USD 570 million across more than 30 instances, since 2017
§ The rising interest of stakeholders in exosome therapeutics is also reflected by the increasing number of partnerships established by various industry and non-industry players
§ Stakeholders have participated in various global events to discuss the research outcomes, and affiliated challenges as well as opportunities existing in this domain
§ At present, more than 30 start-ups are driving innovation in this domain; a variety of R&D initiatives have been undertaken by these players over the last few years for the development of exosome therapeutics
§ Lack of efficacy, COVID-19 pandemic, limited patient enrollment, and scarce funding are among the key reasons that have led to the discontinuation of studies sponsored by various industry and non-industry players
§ With the rising demand for therapeutic advances in drug safety, the market of exosome therapeutics is expected to grow at an annualized rate of 41% between 2029-2040
§ The projected market opportunity is likely to be well distributed across different routes of administration, types of formulations and key geographical regions
Table of Contents
1. PREFACE
1.1. Scope of the Report
1.2. Market Segmentation
1.3. Research Methodology
1.4. Key Questions Answered
1.5. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
3.1. Overview of Exosomes
3.1.1. Types of Extracellular Vesicles
3.1.1. Potential Sources of Exosomes
3.2. Exosome Biogenesis
3.2.1. Exosome Formation and Development Process
3.2.2. Secretion of Exosomes
3.3. Applications of Exosomes
3.4. Mechanism of Exosome Therapy
3.4.1. Exosome Drug Therapy
3.4.2. Exosome RNAi Therapy
3.4.3. Exosome Immunotherapy
3.5. Advantages of Exosome Therapies
3.6. Risks and Future Perspectives Associated with Exosome Therapeutics
4. EXOSOME THERAPEUTICS: MARKET LANDSCAPE
4.1. Analysis Methodology and Key Parameters
4.2. Exosome Therapeutics Market Landscape
4.2.1. Analysis by Phase of Development
4.2.2. Analysis by Technology Platform
4.2.3. Analysis by Type of Payload
4.2.4. Analysis by Derived Source
4.2.5. Analysis by Target Disease Indication(s)
4.2.6. Analysis by Therapeutic Area
4.2.7. Analysis by Phase of Development and Therapeutic Area
4.2.8. Analysis by Therapeutic Area and Route of Administration
4.2.9. Analysis by Route of Administration
4.2.10 Analysis by Area of Application
4.2.11. Analysis by Type of Therapy
4.2.12. Analysis by Dosing Frequency
4.2.13. Analysis by Type of Therapy (By Method of Composition)
4.2.14. Analysis by Line of Treatment
4.3. Exosome Therapeutics Developers
4.3.1. Analysis by Year of Establishment
4.3.2. Analysis by Company Size
4.3.3. Analysis by Location of Headquarters
4.3.4. Most Active Players: Analysis by Number of Therapeutics
5. EXOSOME THERAPEUTICS DEVELOPERS: COMPANY PROFILES
5.1. Coya Therapeutics
5.1.1. Company Overview
5.1.2. Product Portfolio
5.1.3. Recent Developments and Future Outlook
5.2. Codiak BioSciences
5.3.1. Company Overview
5.3.2. Product Portfolio
5.3.3. Clinical Trial Information
5.3.4. Recent Developments and Future Outlook
5.4. Evox Therapeutics
5.4.1. Company Overview
5.4.2. Product Portfolio
5.4.3. Recent Developments and Future Outlook
5.5. Curexsys
5.5.1. Company Overview
5.5.2. Product Portfolio
5.5.3. Recent Developments and Future Outlook
5.6. EV Therapeutics
5.6.1. Company Overview
5.6.2. Product Portfolio
5.6.3. Recent Developments and Future Outlook
5.7. SHIFTBIO
5.7.1. Company Overview
5.7.2. Product Portfolio
5.7.3. Recent Developments and Future Outlook
6. EXOSOME THERAPEUTICS: DRUG PROFILES
6.1. AEGLE Therapeutics
6.1.1. Company Overview
6.1.2. AGLE-102: Product Portfolio
6.1.2.1. AGLE-102: Clinical Trial Information
6.1.3. Recent Developments and Future Outlook
6.2. AVEM Healthcare
6.2.1. Company Overview
6.2.2. Ardoxso: Product Portfolio
6.2.2.1. Ardoxso: Clinical Trial Information
6.2.3. Recent Developments and Future Outlook
6.3. Cellular Biomedicine Group
6.3.1. Company Overview
6.3.2. Financial Information
6.3.3. haMPC-Exos: Product Portfolio
6.3.3.1. haMPC-Exos: Clinical Trial Information
6.3.4. hMSC-Exos: Product Portfolio
6.3.4.1. hMSC-Exos: Clinical Trial Information
6.3.5. Undisclosed Drug 1: Product Portfolio
6.3.5.1. Undisclosed Drug 1: Clinical Trial Information
6.3.6. Recent Developments and Future Outlook
6.4. OBCTCD24
6.4.1. Company Overview
6.4.2. CovenD24: Product Portfolio
6.4.2.1. CovenD24: Clinical Trial Information
6.4.3. Recent Developments and Future Outlook
6.5. ReNeuron
6.5.1. Company Overview
6.5.2. Financial Information
6.5.3. Undisclosed Drug 1: Product Portfolio
6.5.3.1. Undisclosed Drug 1: Clinical Trial Information
6.5.4. Recent Developments and Future Outlook
6.6. Stem Cell Medicine
6.6.1. Company Overview
6.6.2. Undisclosed Drug 1: Product Portfolio
6.6.3. Recent Developments and Future Outlook
7. CLINICAL TRIAL ANALYSIS
7.1. Analysis Methodology and Key Parameters
7.2. Exosome Therapeutics: List of Clinical Trials
7.2.1. Analysis by Trial Status
7.2.2. Analysis by Trial Registration Year
7.2.3. Analysis by Sponsor / Collaborator
7.2.4. Analysis by Trial Registration Year and Type of Study
7.2.5. Analysis by Trial Registration Year and Trial Status
7.2.6. Analysis by Annual Number of Patients Enrolled
7.2.7. Analysis by Study Design
7.2.8. Analysis by Age Category
7.2.9. Analysis by Phase of Development and Trial Status
7.2.10. Analysis by Phase of Development and Patients Enrolled
7.2.11. Most Active Industry Players: Analysis by Number of Registered Trials
7.2.12. Most Active Non-Industry Players: Analysis by Number of Registered Trials
7.2.13. Analysis by Trial Location
7.2.14. Analysis by Trial Status and Geography
7.2.15. Analysis by Trial Status, Patients Enrolled and Geography
8. ACADEMIC GRANT ANALYSIS
8.1. Analysis Methodology and Key Parameters
8.2. Exosome Therapeutics: List of Academic Grants
8.3. Analysis by Year of Grants Awarded
8.4. Analysis by Amount Awarded per Year
8.5. Analysis by Type of Funding Institute Center
8.6. Analysis by Support Period
8.7. Analysis by Purpose of Grant
8.8. Word Cloud Analysis: Emerging Focus Area
8.9. Analysis by Grant Activity Code
8.10. Analysis by Location of Recipient Organizations
8.11. Popular Recipient Organizations: Analysis by Number of Grants
8.12. Popular Recipient Organizations: Analysis by Amount Awarded
8.13. Analysis by Type of Recipient Organization
8.14. Analysis by Study Section
8.15. Analysis by Type of Grant Application
8.16. Analysis by Funding Institute Center and Support Year
9. GLOBAL EVENT ANALYSIS
9.1. Chapter Overview
9.2. Scope and Methodology
9.3. List of Global Events Related to Exosomes
9.3.1. Analysis by Year of Event
9.3.2. Analysis by Event Platform
9.3.3. Analysis by Type of Event
9.3.4. Analysis by Region
9.3.5. Most Active Organizers: Analysis by Number of Events
9.3.6. Most Active Speakers: Analysis by Number of Events
9.3.7. Most Active Industry Participants: Analysis by Number of Events
9.3.8. Most Active Non-Industry Participants: Analysis by Number of Events
9.3.9. Analysis by Designation of Participant
9.3.10. Analysis by Affiliated Department of Participant
9.3.11. Word Cloud Analysis: Evolutionary Trends in Event Agenda / Key Focus Area
10. PARTNERSHIPS AND COLLABORATIONS
10.1. Chapter Overview
10.2. Partnership Models
10.3. Exosome Therapeutics: List of Partnerships and Collaborations
10.3.1. Analysis by Year of Partnership
10.3.2. Analysis by Type of Partnership
10.3.3. Analysis by Year and Type of Partnership
10.3.4. Analysis by Company and Type of Partnership
10.3.5. Analysis by Type of Technology Platform
10.3.6. Analysis by Type of Partner
10.3.7. Most Active Players: Analysis by Number of Partnerships
10.3.8. Word Cloud Analysis: Emerging Focus Areas
10.3.9. Analysis by Target Disease Indication(s)
10.3.10.Analysis by Therapeutic Area
10.3.11.Analysis by Therapeutic Area and Type of Partnership
10.3.12. Regional Distribution of Partnerships
10.3.13. Distribution by Geography
11. FUNDING AND INVESTMENT ANALYSIS
11.1. Analysis Methodology and Key Parameters
11.2. Type of Funding
11.3. Exosome Therapeutics: List of Funding Instances
11.4. Analysis by Year of Funding
11.5. Analysis by Amount Invested
11.6. Analysis by Type of Funding
11.7. Analysis by Type of Funding and Amount Invested
11.8. Analysis by Purpose of Funding
11.9. Analysis by Target Disease Indication(s)
11.10. Analysis by Therapeutic Area
11.11. Most Active Players: Analysis by Number of Funding Instances
11.12. Most Active Players: Analysis by Amount Raised
11.13. Active Investors: Analysis by Number of Funding Instances
11.14. Distribution by Geography
11.15. Summary of Funding Activity
12. START-UP HEALTH INDEXING
12.1. Analysis Methodology and Key Parameters
12.2. Analysis by Pipeline Strength
12.3. Analysis by Pipeline Maturity
12.4. Analysis by Indication Diversity
12.5. Analysis by Number of Partnerships
12.6. Analysis by Financial Support
12.7. Start-up Health Indexing: Roots Analysis Perspective
12.8. Most Active Start-ups
13. CASE STUDY: EXOSOME DEVELOPMENT AND MANUFACTURING SERVICE PROVIDERS
13.1. Chapter Overview
13.2. Exosome Development and Manufacturing Service Providers Landscape
13.2.1. Analysis by Year of Establishment
13.2.2. Analysis by Company Size
13.2.3. Analysis by Location of Headquarters
13.2.4. Analysis by Location of Headquarters and Company Size
13.3. Analysis by Type of Service(s) Offered
13.3.1. Analysis by Method of Isolation
13.3.2. Analysis by Method of Purification
13.3.3. Analysis by Method of Characterization
13.3.4. Analysis by Method of Exosome Manufacturing
13.3.5. Analysis by Scale of Operation
13.3.6. Analysis by Scalability
14. DRUG FAILURE ANALYSIS
14.1. Methodology and Key Parameters
14.2. Exosome Therapeutics: List of Failed Drug Candidates
14.2.1. Analysis by Trial Start and Discontinuation Year
14.2.2. Analysis by Trial Status of Discontinuation
14.2.3. Analysis by Target Disease Indication(s)
14.2.4. Analysis by Route of Administration
14.2.5. Analysis by Type of Sponsor
14.2.6. Analysis by Reasons for Drug Failure
14.2.7. Word Cloud Analysis: Emerging Focus Area
15. MARKET SIZING AND OPPORTUNITY ANALYSIS
15.1. Forecast Methodology and Key Assumptions
15.2. Global Exosome Therapeutics Market, 2029-2040
15.2.1. Exosome Therapeutics Market for Allogeneic Therapy, 2029-2040 (USD Million)
15.2.2. Exosome Therapeutics Market for Autologous Therapy, 2029-2040 (USD Million)
15.2.3. Exosome Therapeutics Market for Degenerative Meniscal Injury, 2031-2040 (USD Million)
15.2.4. Exosome Therapeutics Market for Dystrophic Epidermolysis Bullosa, 2030-2040 (USD Million)
15.2.5. Exosome Therapeutics Market for Fistula Perianal, 2029-2040 (USD Million)
15.2.6. Exosome Therapeutics Market for Retinitis Pigmentosa, 2029-2040 (USD Million)
15.2.7. Exosome Therapeutics Market for Dermatological Disorders, 2030-2040 (USD Million)
15.2.8. Exosome Therapeutics Market for Muscoskeletal Disorders, 2031-2040 (USD Million)
15.2.9. Exosome Therapeutics Market for Ophthalmic Disorders, 2029-2040 (USD Million)
15.2.10. Exosome Therapeutics Market for Rectal Disorders, 2029-2040 (USD Million)
15.2.11. Fistula Tract Exosome Therapeutics Market, 2029-2040 (USD Million)
15.2.12. Intra-articular Exosome Therapeutics Market, 2031-2040 (USD Million)
15.2.13. Intra-ocular Exosome Therapeutics Market, 2029-2040 (USD Million)
15.2.14. Exosome Therapeutics Market Geographical Distribution, 2029-2040 (USD Million)
15.2.15. Erciyes University’s Drug: Sales Forecast, 2029-2040 (USD Million)
15.2.16. Synovial Fluid-Derived Mesenchymal Stem Cells: Sales Forecast, 2031-2040 (USD Million)
15.2.17. AGLE-102: Sales Forecast, 2030-2040 (USD Million)
15.2.18. Trehan University of Medical Sciences’ Drug: Sales Forecast, 2029-2040 (USD Million)
15.2.19. ReNeuron’ Drug: Sales Forecast, 2029-2040 (USD Million)
16. EXECUTIVE INSIGHTS
16.1. Capricor Therapeutics
16.1.1. Company Snapshot
16.1.2. Interview Transcript: Xavier Avat, Chief Business Officer
16.2. Exogenus Therapeutics
16.2.1. Company Snapshot
16.2.2. Interview Transcript: Patricia C. Freire, R&D and Innovation Manager
16.3. ILIAS Biologics
16.3.1. Company Snapshot
16.3.2. Interview Transcript: Soonho Song, Chief Business Officer
17. APPENDIX 1: TABULATED DATA
18. APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/exosome-therapeutics-market.html
You may also be interested in the following titles:
You may also like to learn what our experts are sharing in Roots educational series:
Antiviral Drugs Development: Are we Prepared for the Next Viral Pandemic?
Exploring How Artificial Intelligence Is Transforming Digital Pathology
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415




Comments