Carbon dioxide (CO₂) is considered a greenhouse gas because of its ability to trap heat in the Earth's atmosphere, contributing to the greenhouse effect. Here’s an explanation of why CO₂ is categorized as such:
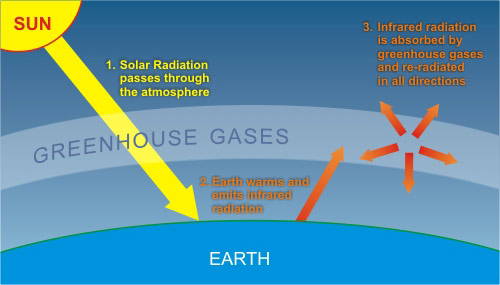
- Absorption of Infrared Radiation: Carbon dioxide molecules have a unique ability to absorb infrared radiation (heat) emitted by the Earth's surface. When the Sun’s energy reaches the Earth, it is absorbed by the surface and re-emitted as infrared radiation.
- Molecular Structure: The molecular structure of CO₂, which consists of one carbon atom and two oxygen atoms, allows it to vibrate and rotate in ways that can absorb and emit infrared radiation. This ability is crucial for its role as a greenhouse gas.
- Contribution to the Greenhouse Effect: The greenhouse effect is a natural process that warms the Earth’s surface. Without it, the planet would be too cold to support most forms of life. Greenhouse gases, including CO₂, methane (CH₄), and water vapor (H₂O), are responsible for this effect. They form a 'blanket' that traps heat, maintaining the Earth's temperature at a habitable level.
- Increased Concentration from Human Activities: Human activities, particularly the burning of fossil fuels such as coal, oil, and natural gas, have significantly increased the concentration of CO₂ in the atmosphere. Deforestation and certain industrial processes also contribute to elevated CO₂ levels.
- Impact on Climate: The increased concentration of CO₂ enhances the greenhouse effect, leading to a rise in global temperatures, commonly referred to as global warming. This warming affects weather patterns, sea levels, and ecosystems.
- Long Atmospheric Lifetime: CO₂ has a relatively long atmospheric lifetime, meaning it remains in the atmosphere for centuries. This long residence time means that CO₂ emissions have long-term effects on the climate system, making current emissions a persistent issue for future generations.




Comments