In today’s highly regulated and competitive industries, particularly within the Life Sciences and Manufacturing sectors, implementing a robust change control process is paramount. A risk-based approach to change control not only ensures compliance with stringent regulatory requirements but also enhances operational efficiency and product quality.
Understanding Change Control in Regulated Industries
The Essence of Change Control
Change control is a systematic process used to manage alterations to products, processes, or systems. In regulated industries like pharmaceuticals and medical devices, change control ensures that any modifications do not negatively impact product quality or compliance. By implementing a structured change control process, companies can mitigate risks, maintain regulatory compliance, and ensure consistent product performance.
Importance of Risk-Based Change Control
A risk-based approach to change control prioritizes changes based on their potential impact on product quality and regulatory compliance. This methodology allows organizations to allocate resources efficiently, focusing on high-risk changes that could significantly affect their operations. By assessing and categorizing changes according to risk, companies can implement more effective control measures, reducing the likelihood of adverse outcomes.
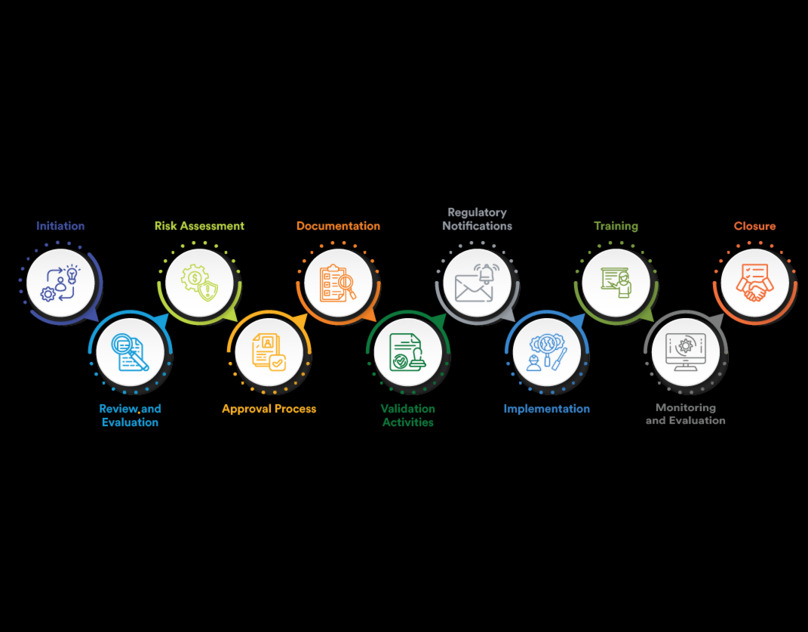
Key Components of a Risk-Based Change Control System
Risk Assessment and Classification
The foundation of a risk-based change control system lies in thorough risk assessment and classification. This involves identifying potential risks associated with a proposed change and evaluating their severity and likelihood. By categorizing changes based on their risk levels, organizations can determine the necessary controls and documentation required for each change.
Documentation and Traceability
Comprehensive documentation is crucial in change control, especially in regulated industries. Every change must be meticulously documented, including the rationale, risk assessment, implementation steps, and verification activities. Traceability ensures that all changes can be tracked and audited, providing a clear history of modifications and their impacts on the product or process.
Stakeholder Involvement
Effective change control requires the involvement of key stakeholders, including quality assurance managers, regulatory affairs teams, and operational personnel. Collaborative decision-making ensures that all aspects of the change are considered, and potential risks are adequately addressed. Engaging stakeholders early in the process fosters accountability and facilitates smoother implementation.
Implementing Change Control in Pharma
Regulatory Requirements in Pharmaceuticals
The pharmaceutical industry is subject to stringent regulatory standards, such as those set by the FDA and EMA. These regulations mandate rigorous change control processes to ensure that any modifications do not compromise product safety, efficacy, or quality. Compliance with these requirements is essential to maintain market authorization and avoid costly regulatory penalties.
Best Practices for Change Control in Pharma
Implementing change control in pharma involves adopting best practices tailored to the industry’s unique challenges. This includes establishing clear procedures, conducting comprehensive risk assessments, ensuring robust documentation, and utilizing specialized software solutions. By adhering to these practices, pharmaceutical companies can enhance their change control processes, ensuring compliance and operational excellence.
Navigating Change Control in Medical Devices
Regulatory Landscape for Medical Devices
Medical device manufacturers must comply with regulations such as ISO 13485 and FDA’s Quality System Regulation (QSR). These standards emphasize the importance of effective change control to ensure that device modifications do not adversely affect safety or performance. A well-structured change control process is critical for maintaining compliance and ensuring the reliability of medical devices.
Strategies for Effective Change Control in Medical Devices
To effectively implement change control in medical devices, companies should focus on thorough risk assessments, clear documentation, and continuous monitoring. Utilizing advanced EDMS software for pharmaceutical regulatory compliance can streamline these processes, providing real-time visibility and enhancing traceability. By adopting these strategies, medical device manufacturers can manage changes efficiently while maintaining high standards of quality and compliance.
Leveraging EDMS Software for Pharmaceutical Regulatory Compliance
Advantages of EDMS Software for Pharmaceutical Regulatory Compliance
Electronic Document Management Systems (EDMS) play a pivotal role in managing change control processes. These systems offer centralized storage, automated workflows, and real-time collaboration, enhancing efficiency and accuracy. For pharmaceutical companies, EDMS Software for Pharmaceutical Regulatory Compliance ensures that all regulatory documentation is easily accessible, up-to-date, and compliant with industry standards. By utilizing specialized EDMS software, organizations can streamline their documentation practices, reduce the risk of errors, and maintain a clear and organized repository of all necessary compliance documents.
Integration with Quality Management Systems
Integrating EDMS Software for Pharmaceutical Regulatory Compliance with broader Quality Management Systems (QMS) provides a seamless approach to managing change control. This integration enables better coordination between different quality processes, such as CAPA (Corrective and Preventive Actions) and audit management. By leveraging EDMS within a comprehensive QMS, organizations can achieve greater coherence and effectiveness in their change control strategies.
Measuring the Effectiveness of Change Control Processes
Key Performance Indicators (KPIs)
To gauge the effectiveness of a risk-based change control approach, organizations should establish and monitor key performance indicators (KPIs). Common KPIs include the number of changes processed, the time taken to implement changes, and the rate of change-related incidents. These metrics provide valuable insights into the efficiency and effectiveness of the change control process.
Continuous Improvement
A successful change control system should incorporate continuous improvement practices. Consistently evaluating and examining change control metrics enables organizations to pinpoint areas for improvement and implement necessary modifications.
Conclusion
As businesses navigate the complexities of regulated industries, implementing a risk-based approach to change control becomes indispensable. ComplianceQuest offers a comprehensive solution that integrates advanced EDMS software for pharmaceutical regulatory compliance with robust Quality Management Systems. By leveraging ComplianceQuest, companies can streamline their change control processes, enhance risk management, and ensure unwavering compliance with regulatory standards. In 2025, staying ahead in a competitive and highly regulated environment will require robust, flexible, and intelligent quality management solutions. ComplianceQuest stands out as an essential tool for businesses aiming to achieve operational excellence, maintain compliance, and drive sustainable growth in the years to come.




Comments