Introduction:
In the world of medical device clinical evaluation development and market approval, clinical evaluations stand as a critical component, demonstrating safety, performance, and efficacy. These evaluations are complex and require a deep understanding of regulatory requirements, clinical evidence, and risk management. Clinical evaluation consultants specialize in navigating this intricate process, offering expertise and strategic guidance. This blog explores the vital role of clinical evaluation consultants, the services they provide, and how they contribute to the successful launch and sustained compliance of medical devices.
Understanding Clinical Evaluations:
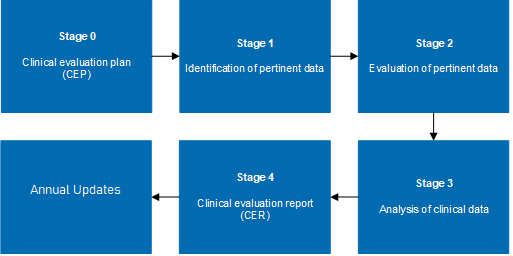
A clinical evaluation is a systematic and ongoing process of appraising clinical data pertaining to a medical device. It involves assessing whether there is sufficient clinical evidence to confirm compliance with relevant essential requirements for safety and performance. This process is crucial not only for initial market approval but also for maintaining compliance as regulations and standards evolve.

The Role of Clinical Evaluation Consultants:
Clinical evaluation consultants are experts in understanding the nuances of regulatory guidelines, clinical research, and evidence synthesis. They play several crucial roles:
- Regulatory Expertise: Consultants stay abreast of the latest regulations and guidelines from bodies like the FDA, EMA, or other national authorities. They understand how these regulations translate into practical strategy and documentation.
- Clinical Evidence Assessment: They conduct thorough assessments of existing clinical data, literature, and reports to determine the safety and performance of a device. This involves a critical evaluation of both favorable and unfavorable data.
- Gap Analysis and Data Collection Strategy: Consultants identify any gaps in clinical evidence and advise on strategies for additional data collection, be it through literature review, post-market surveillance, or clinical investigations.
- Documentation and Reporting: They assist in preparing robust clinical evaluation reports (CERs) and other necessary documentation that meet the scrutiny of regulatory authorities.
- Ongoing Evaluation and Updates: clinical evaluation plan template is not a one-time task but an ongoing process. Consultants ensure that evaluations are updated regularly to reflect any new data, changes in clinical practice, or evolving regulatory requirements.
Benefits of Clinical Evaluation Consultant Services:
- Expert Guidance: With their specialized knowledge, consultants can navigate complex regulatory requirements and ensure that evaluations meet the highest standards of rigor and comprehensiveness.
- Efficiency and Speed to Market: By streamlining the clinical evaluation process, consultants can expedite the overall path to market approval and reduce delays.
- Risk Mitigation: A thorough and well-conducted clinical evaluation significantly reduces the risk of non-compliance and potential market withdrawal or legal issues.
- Focus on Innovation: Freeing manufacturers from the complexities of clinical evaluations, consultants allow them to focus more on innovation and core business operations.
Conclusion:
Clinical evaluation consultants are invaluable allies in the journey of medical device development and market approval. Their expertise, strategic guidance, and ongoing support ensure that clinical evaluations are conducted efficiently, effectively, and in full compliance with regulatory standards. In a rapidly evolving medical landscape, the role of clinical evaluation consultants becomes increasingly crucial. They not only facilitate a smoother approval process but also contribute to the ongoing success and credibility of medical devices in the market. As regulatory requirements become more stringent and the demand for robust clinical evidence grows, the services provided by these consultants will continue to be a cornerstone of successful medical device commercialization.




Comments