Introduction:
Anteris Technologies, an Transcatheter Aortic Valve Implantation (TAVI) Market, is making significant waves in the rapidly evolving field of Transcatheter Aortic Valve Implantation (TAVI). Known for its commitment to innovation, Anteris Technologies is pioneering the development of next-generation heart valve technologies, particularly its unique DurAVR aortic valve. As the global demand for minimally invasive heart valve replacement procedures continues to rise, Anteris Technologies has strategically positioned itself as a leader in the TAVI market by focusing on novel approaches, patient-centric innovations, and strategic growth initiatives. This article explores Anteris Technologies’ strategies, emerging innovations, and developments shaping the future of the TAVI market.
Anteris Technologies: A Company Overview
Founded in 2011, Anteris Technologies is an Australian-based biotechnology company focused on developing advanced cardiovascular devices. The company specializes in the design and commercialization of its DurAVR transcatheter aortic valve, which is designed to offer superior performance and durability for patients suffering from aortic stenosis. Anteris’ focus on innovation, coupled with its commitment to improving patient outcomes, has enabled it to develop cutting-edge technology that stands out in the competitive TAVI space.
While the company has achieved significant milestones with its DurAVR valve, its focus extends beyond just the product. Anteris Technologies is continuously pushing boundaries in terms of device design, clinical trials, and global market penetration, ensuring that its products meet the needs of healthcare providers and patients alike.
Strategic Approach to Growth and Market Penetration
Anteris Technologies’ strategy for dominating the TAVI market revolves around multiple facets, from pioneering innovative product designs to expanding its presence in key global markets. The company’s approach can be broken down into several key areas:
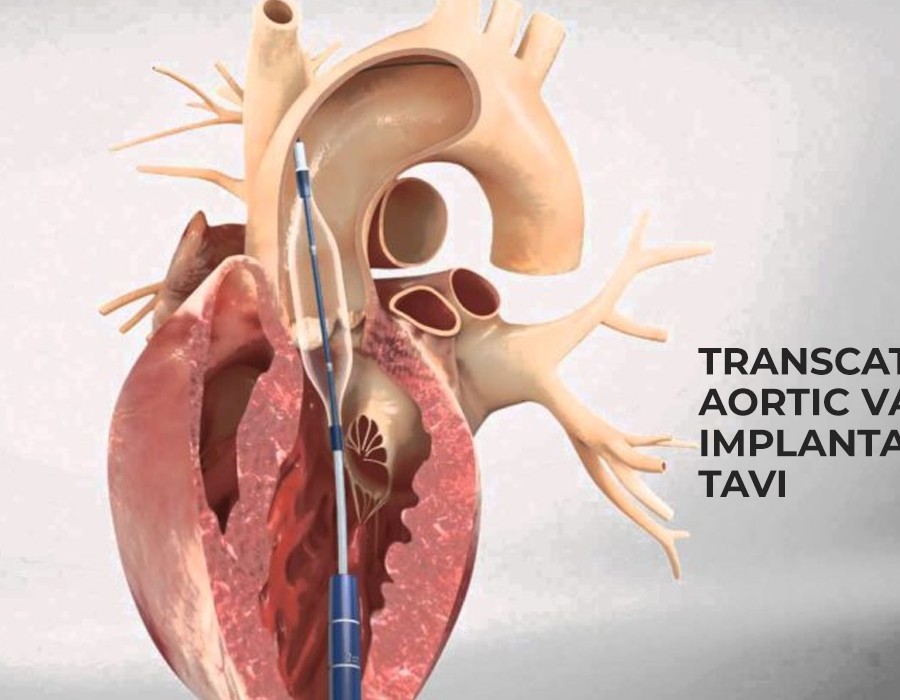
1. Innovative Valve Design: DurAVR’s Competitive Edge
The cornerstone of Anteris Technologies’ strategy lies in its breakthrough product, the DurAVR valve. What sets the DurAVR apart from other TAVI devices is its unique single-piece, 3D-shaped, bovine pericardial tissue valve. This design allows for better durability and flexibility compared to traditional TAVI valves, making it a promising option for long-term valve replacement. The DurAVR is designed to minimize paravalvular leak (PVL), a complication often associated with TAVI procedures.
The DurAVR valve’s design also incorporates a self-expanding frame made from a nitinol alloy, which ensures proper anchoring of the valve while maintaining its flexibility. The combination of these features results in a more efficient and long-lasting valve implant, positioning Anteris Technologies as a competitor to established players in the TAVI market.
Additionally, Anteris has focused on creating a valve that can be deployed with a low-profile delivery system, which is crucial for ensuring ease of use and broadening the number of patients eligible for the procedure. This makes the DurAVR valve ideal for individuals with a range of anatomical challenges, such as smaller arteries or complex valve structures, which might exclude them from traditional TAVI procedures.
2. Clinical Trials and Evidence-Based Approach
Anteris Technologies is highly focused on clinical research and gathering evidence to support the safety and efficacy of its DurAVR valve. The company is conducting multiple clinical trials to prove that its valve can deliver superior outcomes compared to existing options.
The company’s APOLLO trial is one such study that aims to evaluate the clinical benefits of the DurAVR valve in patients with severe aortic stenosis. Early-stage clinical results have shown promising outcomes, including low rates of complications and encouraging patient recovery, which positions Anteris as a potential disruptor in the TAVI market.
Anteris’ commitment to clinical trials not only ensures that its products meet rigorous safety and efficacy standards but also helps to build trust within the medical community. As clinical data supporting the DurAVR valve continues to grow, the company is setting itself up for widespread adoption by healthcare providers around the world.
3. Regulatory Approvals and Market Expansion
For any medical device company, obtaining regulatory approval is a crucial step in bringing products to market. Anteris Technologies is actively working on securing regulatory approvals for its DurAVR valve in key markets, including the United States, Europe, and other regions with a high demand for TAVI solutions.
In particular, the company has made strides in obtaining European CE Mark approval, which allows it to market the DurAVR valve within the European Union. With regulatory approvals in hand, Anteris is now focusing on expanding its market reach in Europe, while simultaneously working toward securing approvals from the U.S. Food and Drug Administration (FDA) for entry into the U.S. market.
To support its global expansion, Anteris is building partnerships with healthcare providers, distributors, and clinicians worldwide. The company has also made efforts to train physicians on how to use its innovative technology effectively, ensuring smooth adoption of the DurAVR valve across a wide range of healthcare systems.
4. Focusing on Minimally Invasive Solutions
As the TAVI field continues to evolve, the trend is toward less-invasive procedures with better outcomes for patients. Anteris Technologies recognizes this shift and has placed a strong emphasis on ensuring that its products are designed for minimally invasive implantation.
The DurAVR valve, which is designed to be implanted through a catheter-based delivery system, allows patients to undergo treatment with smaller incisions, reduced risks of complications, and faster recovery times compared to traditional open-heart surgery. This is particularly important for elderly patients or those with comorbidities who may not be candidates for more invasive procedures.
By focusing on minimally invasive techniques, Anteris is aligning itself with the broader trend in healthcare toward procedures that improve patient comfort, reduce healthcare costs, and shorten hospital stays.
Emerging Innovations and Developments
Anteris Technologies is not content with just developing its DurAVR valve. The company is actively investing in innovation and continuously exploring new frontiers in heart valve replacement therapies. Some of the most notable emerging developments from Anteris include:
1. Next-Generation DurAVR Valve: Expanded Indications and Design Enhancements
Building on the success of its initial DurAVR valve, Anteris is working on future iterations of the valve with expanded indications and further improvements to design and performance. The company plans to optimize its valve to accommodate a broader range of patient anatomies, particularly those with challenging or complex valve structures. These new iterations could make TAVI even more accessible to patients previously excluded from the procedure.
Additionally, Anteris is focused on improving long-term valve durability, a crucial factor in ensuring the success of TAVI procedures for aging populations. The company is exploring ways to enhance tissue durability, reduce the risk of valve degeneration, and provide patients with longer-lasting valve replacements.
2. Technological Integration: Advanced Imaging and Monitoring Systems
Anteris Technologies is exploring the integration of its valve technology with advanced imaging and monitoring systems. Real-time imaging solutions allow for more precise valve placement and positioning during TAVI procedures, which is critical for achieving optimal clinical outcomes. By leveraging cutting-edge technologies such as 3D imaging, Anteris aims to reduce procedural errors and improve overall patient safety.
Moreover, the company is looking into post-procedure monitoring tools that can help track the performance of the DurAVR valve in real-time, enabling healthcare providers to better manage patient recovery and address complications before they become serious.
Future Outlook: Anteris Technologies in the TAVI Market
As the global TAVI market continues to expand, Anteris Technologies is well-positioned to capitalize on the growing demand for innovative, minimally invasive heart valve solutions. With its strong focus on the DurAVR valve, the company is not only providing an alternative to traditional valve replacement procedures but also reshaping the landscape of heart valve treatments.
In the coming years, Anteris Technologies is likely to play an increasing role in the TAVI market, driven by its innovative product pipeline, strategic regulatory efforts, and commitment to improving patient outcomes. As the company moves toward broader global adoption, it will continue to be a key player in advancing the future of heart valve replacement therapies.
Conclusion
Anteris Technologies is poised to become a significant force in the TAVI market, thanks to its innovative DurAVR valve, focus on minimizing invasiveness, and commitment to continuous clinical research. With ongoing product development, expanding market reach, and promising clinical trial results, Anteris is on track to make a lasting impact on the TAVI space, improving the lives of millions of patients worldwide suffering from aortic stenosis and other valve diseases. As the company continues to innovate and expand, its role in reshaping the heart valve therapy landscape is becoming increasingly clear.




Comments