Hepatitis E virus (HEV) is classified in the genus Orthohepevirus, family Hepeviridae. HEV is one of five known human hepatitis virus (A, B, C, D, and E) and causes the hepatitis E. Most people with hepatitis E recover completely. During hepatitis E outbreaks, the overall case-fatality rate is about 1%. However, for pregnant women, hepatitis E can be a serious illness, with mortality reaching 10%–30% among pregnant women in their third trimester. HEV can be clustered genetically into 4 genotypes, and genotypes 3 and 4 tend to be the ones that cause chronic hepatitis in the immunosuppressed. The HEV has a fecal-oral transmission route and can spread by the consumption of uncooked or undercooked meat or fecally contaminated water within endemic areas. In 2017, hepatitis E was estimated to affect more than 19 million people. Hepatitis E is most common in developing countries with inadequate water supply and poor environmental sanitation. A preventive vaccine (HEV 239) is approved for use in China and no hepatitis E vaccine is licensed for use in the United States.
HEV is a small, positive-sense single-stranded RNA virus with an icosahedral capsid. HEV virions exist in two forms in the infected host, non-enveloped (neHEV) and quasi-enveloped (eHEV) particles. Virions secreted in feces are non-enveloped, spherical particles of approximately 27–34 nm in diameter. However, virions secreted in circulating blood and supernatant of infected cell cultures are quasi-enveloped as they are covered with a lipid envelope. Although neHEV particles are more infectious, eHEV particles are resistant to antibody neutralization against the viral capsid protein.The genome of HEV is a positive-sense, single-stranded RNA that is approximately 7.2k bases length, which contains 3 open reading frames (ORFs,1-3), and 5' and 3' cis acting elements. ORF1 encodes a nonstructural polyprotein for virus replication and transcription; ORF2 encodes the capsid protein that assemble into virion particles, binds host cells, and elicit neutralizing antibodies; ORF3, which partially overlaps ORF2, encodes a multifunctional protein involved in virion morphogenesis and pathogenesis.
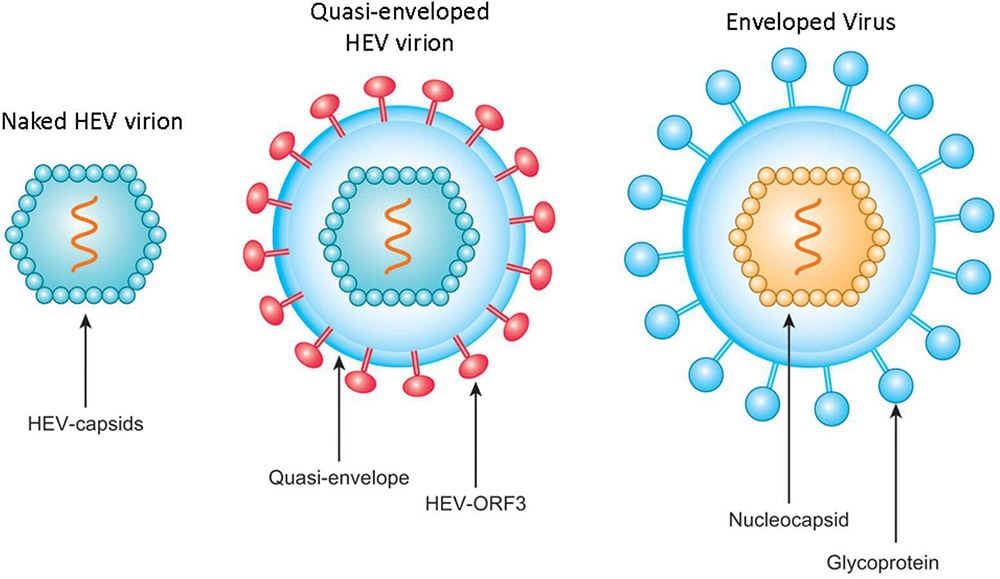
Fig.1 Schematic illustration of non-enveloped and quasi-enveloped HEV particles as well as enveloped virus1
As a fecal-orally transmitted virus, HEV first enters the host via the gastrointestinal tract and replicates in intestinal epithelial cells. Subsequently, the virus enters the bloodstream via viremia and reaches its target organ, the liver. Nonenveloped HEV virions bind to cellular receptors. Heparin sulfate proteoglycans (HSPGs) and heat shock cognate protein 70 (HSC70) are thought to be the attachment receptors for HEV but more research is required to determine the entry receptors for HEV. Quasi-enveloped HEV (eHEV) particles enters liver cells via dynamin-dependent, clathrin-mediated endocytosis, which requires Rab 5 and Rab7 GTPases and lysosome function before uncoating within the host cell. The viral genomic RNA is released to cytosol after uncoating of capsid protein with an unknown process. The viral nonstructural proteins within ORF1 transcribe the positive-sense RNA genome into a negative-strand intermediate, which serves as messenger for translation of nonstructural proteins and ORF2 and ORF3 proteins. Finally, genomic RNA is packaged into progeny HEV virions and secreted outside the cell. Figure 2 indicates the proposed life cycle of hepatitis E virus (HEV).

Fig. 2 Proposed life cycle of HEV2
Creative Diagnostics now can provide our customers plenty Hepatitis E virus (HEV) antigens for different applications. We always try our best to provide you high-quality products with cheaper price.
References
- Nan, Y.; et al. (2018). Vaccine Development against Zoonotic Hepatitis E Virus: Open Questions and Remaining Challenges. Frontiers in Microbiology, 9.
- Wang, B.; Meng, X.J. (2021). Structural and molecular biology of hepatitis E virus. Computational and Structural Biotechnology Journal, 19, 1907–1916.




Comments