In today’s highly regulated industries, particularly within the Life Sciences and Manufacturing sectors, effective Compliance Management is not just a necessity but a cornerstone of business success. Properly documenting compliance efforts ensures that organizations adhere to stringent regulatory standards, maintain quality assurance, and avoid costly penalties.
Understanding Compliance Management
Definition and Scope of Compliance Management
Compliance Management encompasses the processes and procedures an organization adopts to ensure adherence to external regulations and internal policies. It involves identifying applicable laws, implementing necessary controls, and continuously monitoring activities to maintain compliance. In sectors like Medical Devices and Pharmaceuticals, where regulatory scrutiny is intense, effective Compliance Management is crucial for sustaining operational integrity and market credibility.
The Role of Compliance Management in Quality Assurance
Quality Assurance (QA) is intrinsically linked to Compliance Management. By systematically documenting compliance efforts, organizations can demonstrate their commitment to maintaining high-quality standards. This integration ensures that products meet regulatory requirements and consumer expectations, fostering trust and reliability in the marketplace.
Regulatory Compliance Management: An Overview
Key Regulations Impacting the Life Sciences and Manufacturing Sectors
The Life Sciences and Manufacturing sectors are governed by a myriad of regulations aimed at ensuring product safety, efficacy, and quality. For instance, the 21 CFR Part 11 Compliance mandates electronic records and signatures to be trustworthy and reliable. Similarly, manufacturing companies must comply with standards such as ISO 9001 and industry-specific regulations. Understanding and navigating these regulations is a fundamental aspect of regulatory compliance management, ensuring that organizations operate within legal frameworks.
Navigating the Complexities of Regulatory Compliance Management
Regulatory Compliance Management involves staying abreast of evolving regulations and adapting organizational practices accordingly. This requires a proactive approach to monitor regulatory changes, assess their impact, and implement necessary adjustments. Leveraging advanced document management systems can streamline this process, enabling efficient tracking and updating of compliance-related documents. Effective regulatory compliance management not only ensures legal adherence but also enhances operational efficiency and reduces the risk of non-compliance.
Appreciating the Critical Role of 21 CFR Part 11 Compliance
Safeguarding Data Integrity and Security
Adhering to 21 CFR Part 11 is crucial for organizations operating within regulated industries that manage electronic records and digital signatures. This compliance ensures that data integrity and security are maintained, providing a trustworthy framework for handling sensitive information and meeting stringent regulatory standards.
Implementing 21 CFR Part 11 Compliance in Your Organization
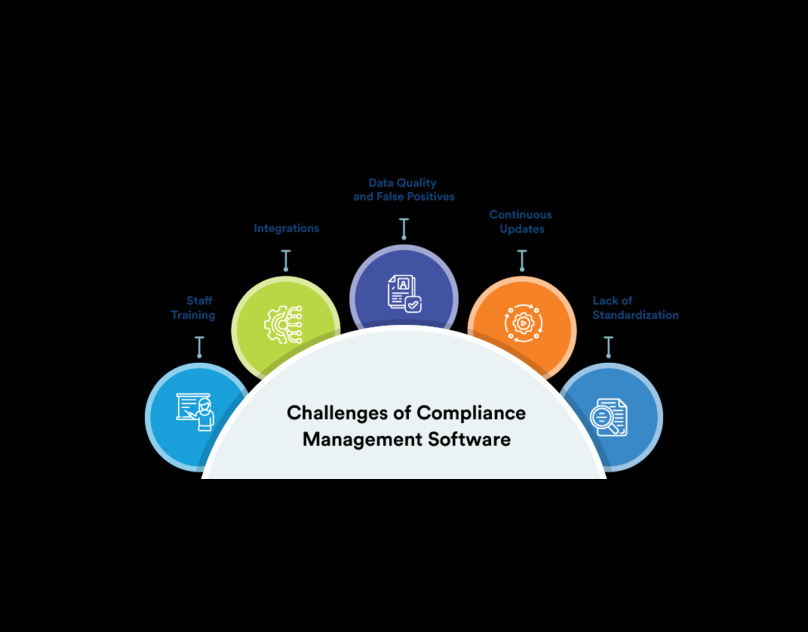
Achieving 21 CFR Part 11 Compliance requires a strategic approach to document management and Compliance Management. Organizations must establish stringent controls over electronic records, including user authentication, audit trails, and data encryption. Utilizing specialized compliance management software can facilitate the implementation process, providing automated tools for monitoring and maintaining compliance.
Document Management: The Backbone of Compliance Efforts
Best Practices in Document Management for Compliance
Effective document management is fundamental to successful Compliance Management. Best practices include maintaining organized and accessible records, implementing version control, and ensuring that all documentation is accurate and up-to-date. Regular audits of document management systems help identify gaps and areas for improvement, ensuring continuous compliance.
Leveraging Technology for Efficient Document Management
Advanced document management systems offer powerful tools to streamline compliance processes. Features such as automated workflows, real-time collaboration, and secure cloud storage enhance the efficiency and reliability of documentation practices. Integrating these systems with Compliance Management platforms provides a cohesive solution for managing regulatory requirements, reducing manual errors, and accelerating compliance reporting. By leveraging technology, organizations can achieve greater accuracy and efficiency in their document management practices.
Building an Effective Compliance Management System
Components of a Robust Compliance Management System
A robust Compliance Management system comprises several key components, including risk assessment, policy development, training programs, and monitoring mechanisms. These elements work together to create a comprehensive framework that supports regulatory adherence and operational excellence. Incorporating document management into this system ensures that all compliance-related information is systematically recorded and easily retrievable, facilitating seamless audits and inspections.
Integrating Compliance Management with Existing Quality Systems
Integrating Compliance Management with existing quality systems enhances overall organizational efficiency and effectiveness. This integration allows for the seamless flow of information between quality assurance processes and compliance efforts, ensuring that both areas are aligned and mutually supportive.
The How-To Guide: Steps to Document Compliance Efforts
Step 1 – Assessing Compliance Requirements
The first step in documenting compliance efforts involves a thorough assessment of applicable regulations and standards. This includes identifying industry-specific requirements, such as 21 CFR Part 11 Compliance for Life Sciences companies or ISO standards for manufacturing firms. Conducting a gap analysis helps determine current compliance status and highlights areas that require improvement.
Step 2 – Developing Documentation Processes
Once compliance requirements are identified, organizations must develop standardized documentation processes. This involves creating templates, defining documentation protocols, and establishing clear guidelines for record-keeping. Effective document management practices ensure that all compliance-related information is consistently captured and organized, facilitating easy access and retrieval during audits.
Step 3 – Implementing Compliance Documentation Tools
Implementing specialized compliance documentation tools is essential for streamlining and automating compliance efforts. These tools offer features such as automated record-keeping, real-time monitoring, and customizable reporting, which enhance the efficiency and accuracy of Compliance Management. By leveraging advanced document management solutions, organizations can reduce manual workloads, minimize errors, and ensure that all compliance documentation is up-to-date and readily available.
Conclusion
Documenting compliance efforts is a critical aspect of Compliance Management that ensures organizations meet regulatory requirements, maintain high-quality standards, and mitigate risks. In the dynamic landscape of 2024, having a robust and integrated compliance management system is essential for businesses to thrive in highly regulated and competitive industries. ComplianceQuest stands out as an indispensable tool for modern businesses, offering comprehensive solutions that streamline regulatory compliance management, enhance document management, and support 21 CFR Part 11 Compliance.




Comments