Effective change management is crucial in the highly regulated industries of life sciences, pharmaceutical, medical devices, and complex manufacturing sectors like aerospace and defense. Facilitating successful Change Management workshops can help organizations navigate transformations smoothly and ensure that key stakeholders are aligned. Whether your focus is improving quality systems, integrating new technologies, or refining CAPA programs, a structured approach to change management workshops can make a significant difference.
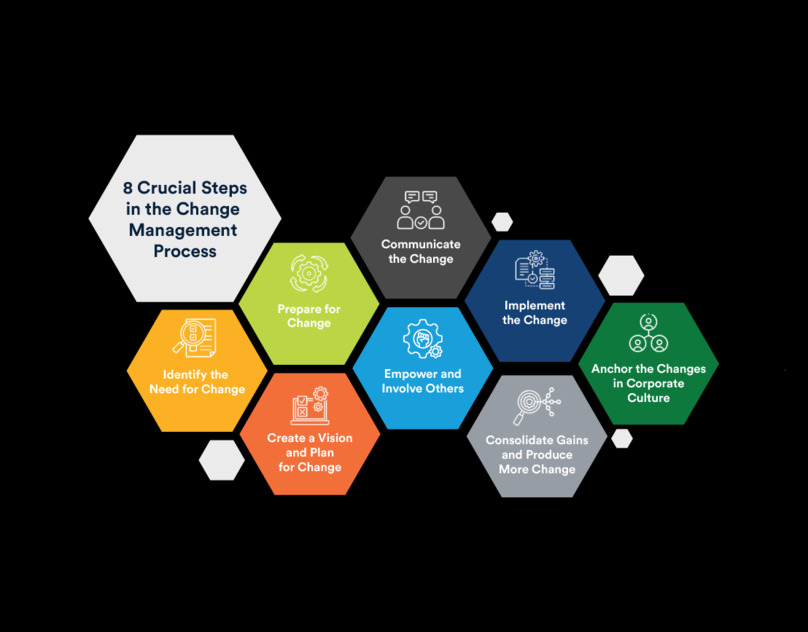
Understanding the Core Principles of Change Management
Workshops on Change management should begin with a clear understanding of the fundamental principles. The key is to ensure participants grasp the importance of alignment across teams, effective communication, and the role of leadership in driving the change. Leaders in sectors such as pharmaceutical and medical devices, where compliance is critical, must emphasize that change management is more than just a one-time effort; it’s an ongoing process supported by change management software and CAPA programs.
During the workshop, introducing the purpose of change management can help set the stage. Leaders can explain how Change Management Software assists in tracking progress, maintaining regulatory compliance, and identifying areas for improvement through CAPA.
Preparing for a Successful Change Management Workshop
Preparation is essential for a change management workshop to run smoothly. Before the session, ensure that the right stakeholders are involved, including representatives from different departments such as quality assurance, regulatory affairs, and manufacturing. It’s important to tailor the content to the specific needs of the organization, whether it’s implementing a new quality system, transitioning to digital platforms, or optimizing CAPA processes.
Leaders should prepare an agenda that covers key topics like change management software implementation, CAPA program optimization, and regulatory considerations specific to the life sciences and manufacturing sectors. The goal is to ensure that participants leave with a clear roadmap for implementing changes in their respective areas.
Engaging Participants Through Interactive Discussions
A change management workshop should not be a one-way lecture. Instead, it should involve interactive discussions that allow participants to share their perspectives and experiences. Leaders should encourage open dialogue about current challenges within their quality systems, CAPA programs, and overall change management processes. This fosters a collaborative environment where solutions can be developed in real-time.
When discussing tools like change management software, it's essential to highlight real-world applications. Participants should understand how such software can automate workflow processes, streamline documentation, and enhance regulatory compliance in industries like pharmaceuticals and medical devices.
Utilizing Case Studies and Real-World Examples
One of the most effective ways to facilitate a change management workshop is through the use of case studies. Real-world examples can provide valuable insights into the challenges and successes of implementing change management software, refining CAPA programs, and driving organizational change. For leaders in the life sciences and complex manufacturing industries, case studies can demonstrate how others have successfully navigated similar challenges.
Leaders can present case studies focusing on companies in the medical device or pharmaceutical sectors that have utilized change management software to streamline CAPA processes, ensuring that the organization stays compliant with stringent regulations.
Encouraging Cross-Departmental Collaboration
Change management workshops should highlight the importance of cross-departmental collaboration. Successful change initiatives often require input from multiple departments, including quality assurance, production, regulatory affairs, and IT. In industries like life sciences and manufacturing, this collaboration is critical to ensure that changes are implemented in a way that meets both operational and regulatory requirements.
Leaders can discuss how CAPA programs benefit from a holistic approach, where quality management is integrated with other business processes through advanced change management software.
Facilitating Hands-On Exercises
Workshops are most effective when participants can engage in hands-on exercises. Leaders should include activities that allow attendees to practice using change management tools or apply CAPA methodologies. For instance, participants could work in small groups to identify potential issues in a CAPA program and then map out steps to resolve them using change management software.
These exercises not only reinforce the material covered but also help participants understand how to apply these concepts in their day-to-day work. Hands-on experience is particularly valuable in industries like medical devices and pharmaceuticals, where understanding the practical application of change management tools is crucial to maintaining compliance.
Managing Resistance to Change
Resistance to change is a common challenge in any organization, especially in highly regulated industries like pharmaceuticals and aerospace. A critical part of any change management workshop is addressing resistance and helping leaders understand how to manage it effectively. This requires a focus on communication, empathy, and providing clear reasons for the change.
Leaders should provide guidance on how to leverage change management software to communicate updates, track stakeholder engagement, and address concerns as they arise. In CAPA programs, this can mean using data to show how changes will improve quality and compliance outcomes.
Measuring the Success of Change Management Initiatives
No change management workshop is complete without a discussion on how to measure success. Leaders need to ensure that they have the right metrics in place to track the impact of their change management efforts. This includes using change management software to monitor key performance indicators (KPIs) related to CAPA programs, quality management systems, and overall organizational performance.
Participants should leave the workshop with a clear understanding of how to measure the effectiveness of their change initiatives, whether through improvements in CAPA compliance, increased efficiency in quality management processes, or better regulatory adherence.
Conclusion
As industries like life sciences and manufacturing continue to evolve, the importance of staying ahead with robust change management processes cannot be overstated. ComplianceQuest’s solutions offer an integrated platform for managing change management, CAPA programs, and quality systems, ensuring organizations remain compliant and competitive in a rapidly changing landscape. By using advanced tools like change management software, companies can drive efficiency, enhance collaboration, and ensure continuous improvement.




Comments